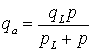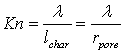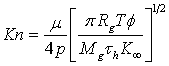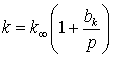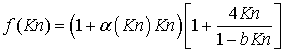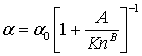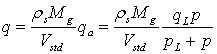Single-phase compressible gas flow through porous media is represented by augmenting the standard porous flow equations so that several additional mechanisms are represented. These include:
1 Fully compressible treatment of gas phase via a gas equation of state.
2 A flow model that accommodates a wide range of fundamental flow mechanisms; i.e. continuum, slip, transition, and free molecular flow, depending on the Knudsen number.
3 Adsorption/desorption model for transfer of gas between the shale and gas phases.
The real-gas equation of state is defined as:
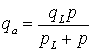
where
|
Z
|
|
is the real gas deviation factor (dimensionless) which may be dependent on pressure Z ≤ 1.0
|
|
T
|
(K)
|
is the temperature,
|
|
Mg
|
(kg/kmol)
|
is the molecular mass of the gas
|
|
Rg
|
(J/mol/K)
|
is the universal gas constant.
|
Representation of isothermal gas adsorption/desorption using the Langmuir isotherm requires specification of the following parameters in the Fluid_properties data structure:
Equation_state_type Integer_value
Equation_state_properties IDM=2
Real_value
Real_value
Gas_universal_constant Real_value
Molecular_weight Real_value
|
|
Flow mechanisms vary according to Knudsen number (Kn), which is a measure of the degree of rarefaction of gas flow through small pores, and is defined as:
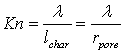
where
|
λ
|
is the mean free path of molecules
|
|
lchar
|
is the representative path; e.g. the mean hydraulic radius rpore.
|
Kinetic theory for a perfect gas may be used to derived expressions for λ. For example the expression adopted by Civen et al. (2011) leads to
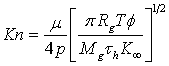
where
|
μ
|
Pa s
|
is the viscosity of gas
|
|
p
|
Pa
|
is the absolute gas pressure
|
|
Rg
|
J/kmol/K
|
is the universal gas constant
|
|
T
|
K
|
is the absolute temperature
|
|
Mg
|
kg/kmol
|
is the molecular mass of gas
|
|
τh
|
|
is the tortuosity
|
The Knudsen number relates pore structure to flow regime, which can be visualized for a shale-gas formation in the figure below. This shows that Darcy flow is usually dominant in the high-permeability fractured zone adjacent to the wellbore, whereas free molecular or transition flow may be dominant provided the porosity of the shale is sufficiently low.
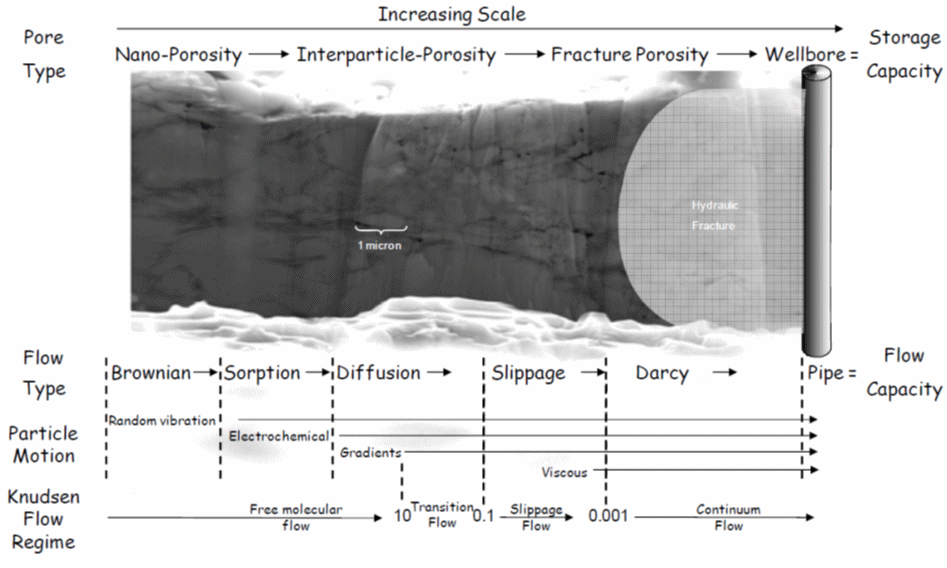 Gas-Shale Stoage and Flow Capacity Diagram (Sondergeld et al., 2010) The flow regimes is also dependent on gas pressure, as the Knudsen number is inversely proportional to gas pressure. This is evident from the plot of Knudsen number vs. gas pressure below, where the typical conditions found in conventional, tight-gas and shale-gas reservoirs are also shown (Freeman et al., 2011)
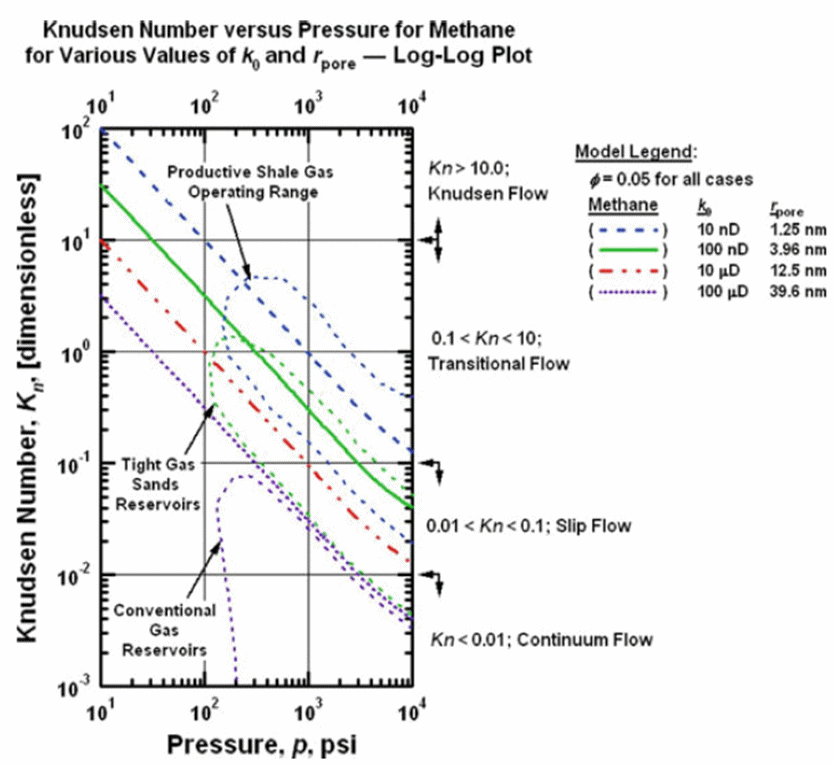 Flow Regimes in Tight-Gas and Shale-Gas Reservoirs (Freeman et al., 2011) Four types of flow regimes are generally identified for different Knudsen number ranges:
Flow Regime
|
Knudsen Range
|
Description
|
Continuum
|
(Kn < 0.01)
|
The mean free path of the gas molecules is negligible compared to the characteristic dimension of the flow geometry
|
Slippage Flow
|
(0.01 < Kn < 0.1)
|
The slippage phenomenon occurs in the layer of molecules immediately adjacent to the boundary walls
|
Transition Regime
|
(0.1 < Kn < 10)
|
|
Molecular Flow Regime
|
(Kn > 10)
|
The flow is dominated by diffusive effects
|
|
Gas slippage is generally represented by an effective permeability law which may be written in the form:
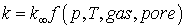
where k∞ is the absolute permeability of the sample. Several different forms of this model are implemented including:
•The Klinkenberg Model (Klinkenberg, 1941) •The Beskok and Karniadakis Model ( Beskok and Karniadakis, 1999)
The Klinkenberg model Klinkenberg (1941) provides a first order correction for gas slippage the equivalent permeability (k) is defined as:
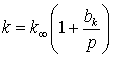
where
|
k∞
|
is the absolute permeability
|
|
bk
|
is the gas slippage factor which is a function of the mean free path (λ) of the molecules at the mean pressure (p) and effective pore radius (r)
|
The data input required for the Klinkenberg model is
Knudsen_diffusion_type 1
Knudsen_diffusion_data IDM=1
Real_value
|
|
Beskok and Karniadakis (1999) derived a second order accurate expression for permeability by studying rarefied gas flows in channels, pipes, and ducts with smooth surfaces in a wide range of Knudsen number (Kn) at low Mach number (M). They propose an expression of the form
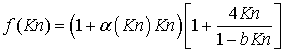
where
|
b
|
is the slippage coefficient (Civan et al., 2011), which is different from bk in the Klinkenberg model, and generally assumed to have a value of -1
|
|
α
|
is the rarefaction coefficient
|
The rarefaction coefficient (α) varies in the range 0 < α < α0 for 0 < Kn < ∞ (Beskok and Kaniadiakis, 1999) and Civan et al. (2011) suggests a relationship of the form
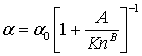
where A and B are empirical constants. These constants must be determined either numerically or experimentally by regression analysis of flow at different Knudsen number for each given class of channel geometry, as A and B depend on the flow channel shape. An extensive discussion on the determination of α is presented by Beskok and Kaniadiakis (1999).
The data input required for the Beskok and Karniadakis model is:
Knudsen_diffusion_type 2
Knudsen_diffusion_data IDM=4
Real_value
Real_value
Real_value
Real_value
|
Additionally the following must be defined to enable evaluation of the Knudsen Diffusion coefficient
Totuosity Real_value
Gas_universal_constant Real_value
Molecular_weight Real_value
|
|
|
The Langmuir isotherm (e.g. Civan et al., 2011; Qu et al., 2012) relates the coverage or adsorption of molecules on a solid surface to gas pressure or concentration of a medium above the solid surface at a fixed temperature. The Langmuir isotherm is defined as:
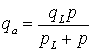
which can be expressed as
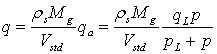
where
|
ρs
|
(kg/m3)
|
is the grain density
|
|
q
|
(kg/m3)
|
is the mass of gas adsorbed per solid volume
|
|
qa
|
(std m3/kg)
|
is the standard volume of gas adsorbed per solid mass
|
|
qL
|
(std m3/kg)
|
is the Langmuir gas volume
|
|
Vstd
|
(std m3/kmol)
|
is the molar volume of gas at standard temperature (273.15K) and pressure (101.325Pa)
|
|
p
|
(Pa)
|
is the gas pressure
|
|
pL
|
(Pa)
|
is the Langmuir gas pressure
|
|
Mg
|
(kg/kmol)
|
is the molecular weight of gas
|
Representation of isothermal gas adsorption/desorption using the Langmuir isotherm requires specification of the following parameters in the Fluid_properties data structure:
Adsorption_type 1
Langmuir_volume real_value
Langmuir_pressure real_value
Std_molar_volume real_value
Molecular_weight real_value
|
|